We have taken up the challenge to manufacture reagents and powders in bulk scale to meet the critical requirements of several industries that we serve. SRL currently offers more than 2500 PRODUCTS and supports its customers in all phases of the drug manufacturing process, from research, quality control and commercial production, through to providing service in concrete safety and regulatory issues.

We are pleased to investigate and provide a quote for your requirements for any quantity; from milligrams to tons. For Custom Synthesis a realistic minimum order volume depends on many factors, but in principal SRL can help you start with small quantities for laboratory testing, scale up for development, and finally go on to economical continuous manufacturing on the ton scale.












This is the first step in the BMCS Business, where we value all communications we have with you and make sure that there is clear, transparent & ethical practice of 100% confidentiality with the information you will share with SRL. Irrespective of whether we have a formal secrecy agreement in place with you, SRL will handle your enquiry and other information with the utmost confidentiality. Should you wish to have a formal agreement put in place before disclosing any details, we will be pleased to arrange this.
We examine your requirements based on your own disclosed technical information. If we feel that additional chemistry or analytical testing is required we will discuss this with you and allow for any extra work in the quotation.
Where you have no synthetic information about the product required, SRL will use its skills to explore options. Having carefully reviewed these, we will then provide a quotation based on our in-house expertise.
SRL‘s Bulk Manufacturing and Custom Synthesis (BMCS) business has broadened its production at our Taloja MIDC site to include existing and new bulk fine chemicals which are being manufactured at commercial scales. Production of the raw materials have been on since 2011 at this site and complements the existing offering of highly regulated active and non-active pharmaceutical ingredients manufactured here.
Most of the products manufactured at the Taloja MIDC bulk plant facility meet and exceed IP, BP, USP & Ph. Eur regulation; follow Current Good Manufacturing Practices (cGMPs) for biopharmaceutical production; and comply with International Consortium for Innovation and Quality in Pharmaceutical Development.

Reaction Capabilities
Equipment
Supporting Utilities at Manufacturing Plant
We can provide raw materials for various type of Industries some are mentioned below
SRL not only provides the raw materials through its BMCS program, but we also cater to the strict documentation requirements which our customers ask. Since the last decade, India has become a hub for the regulated as well as the non-regulated pharmaceutical markets, and to serve them we offer complete VQ documents with all the technical, commercial and ethical information that we can provide.
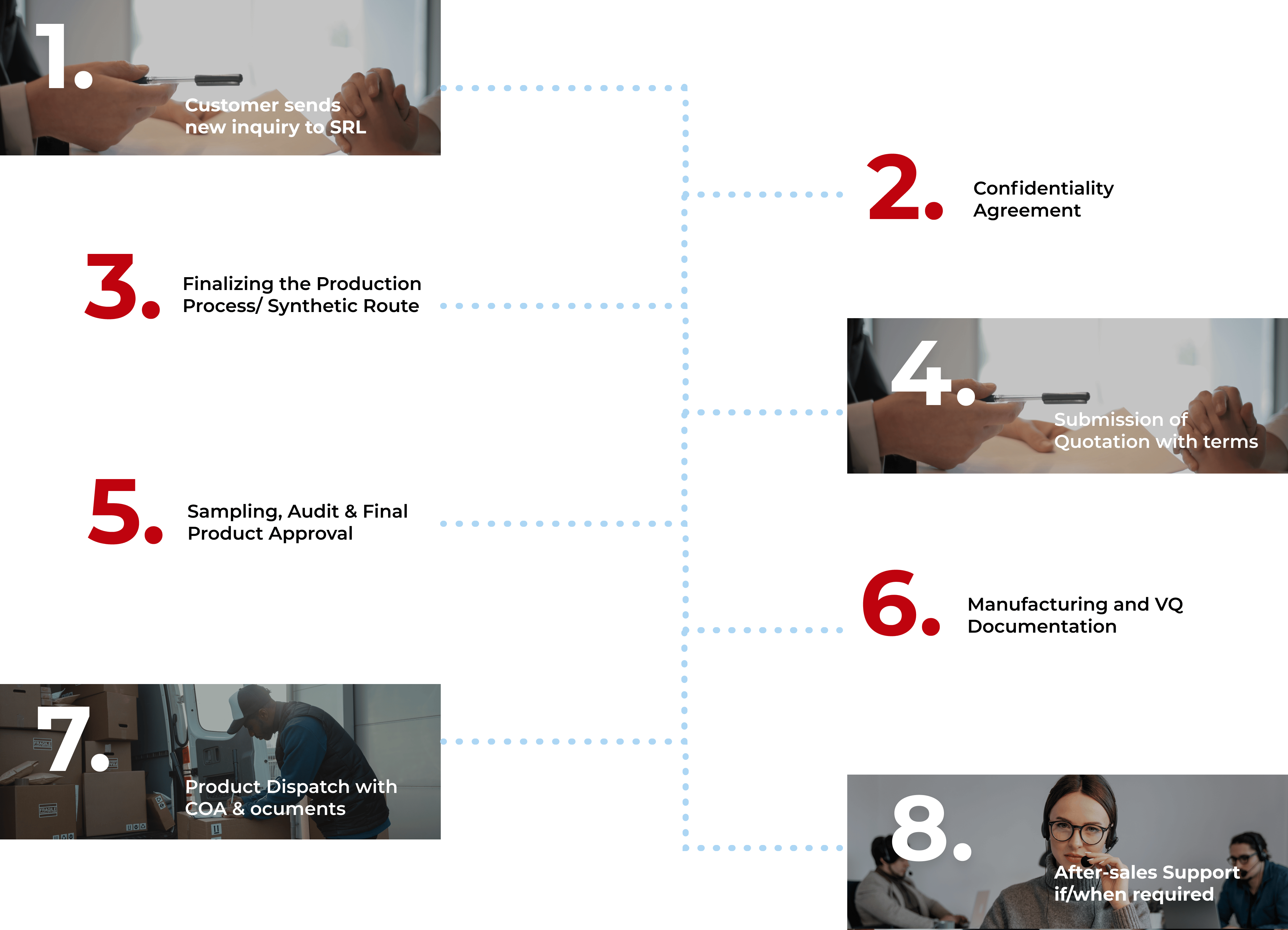
Customer sends new inquiry to SRL
Finalizing the production Process/Synthetic Route
Sampling, Audit & Final Product Approval
Product Dispatch with COA & Documents

Confidentiality Agreement
Submission of Quotation with Terms
Manufacturing and VQ Documentation
After-sales Support if/when required